Physical Chemistry Collection
"Exploring the Fascinating World of Physical Chemistry: From Elements to Emission Spectra" Physical chemistry
All Professionally Made to Order for Quick Shipping
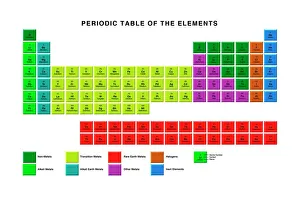
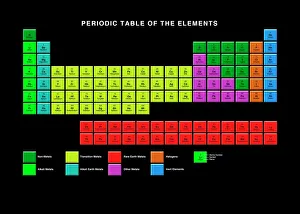
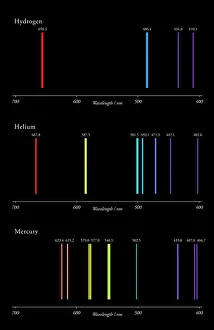
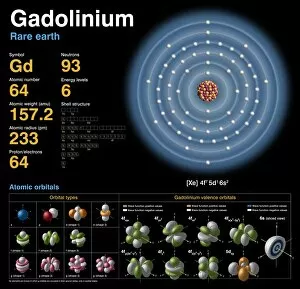
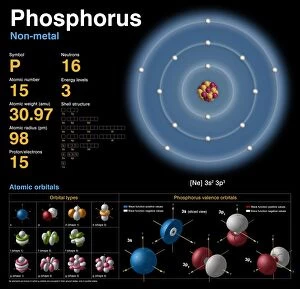
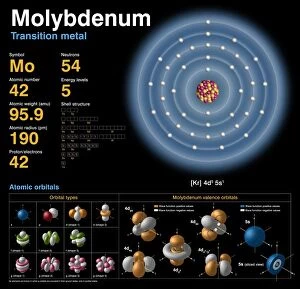
"Exploring the Fascinating World of Physical Chemistry: From Elements to Emission Spectra" Physical chemistry, a captivating field that delves into the intricate relationship between matter and energy. At its core lies the standard periodic table, an invaluable tool showcasing the diverse array of element types that make up our universe. From hydrogen (H) to helium (He), from mercury (Hg) to their mesmerizing emission spectra, physical chemistry unravels the secrets hidden within these atomic building blocks. The work of brilliant minds like John Dalton and Joseph Priestley has paved the way for our understanding of these elements' properties and behaviors. The journey through physical chemistry takes us back in time, as we explore "The Sceptical Chymist, " a groundbreaking publication by Robert Boyle in 1661. Fast forward to c1908 when Ernest Rutherford, a Nobel prize-winning atomic physicist, revolutionized our knowledge with his remarkable discoveries. Even notable figures like John Hall Gladstone, President of the Chemical Society in 1880, have contributed their expertise to this ever-evolving discipline. Artists such as Lock & Whitfield captured their essence through stunning caricatures. Wilhelm Ostwald's contributions cannot be overlooked either; this German physical chemist left an indelible mark on the field with his pioneering research. Physical chemistry is not merely confined within laboratories; it permeates every aspect of our lives. It enables us to comprehend chemical reactions occurring around us daily and provides insights into how substances interact at a molecular level. So let's embark on this enthralling journey together – exploring physical chemistry's depths while marveling at its profound impact on our world.