Carbon Collection (page 8)
Carbon is the unsung hero of our modern world, with its versatility and strength making it an essential element in various industries
All Professionally Made to Order for Quick Shipping
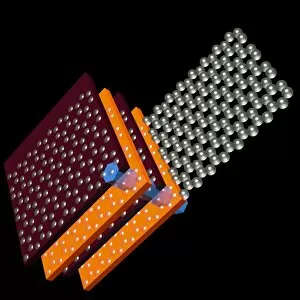
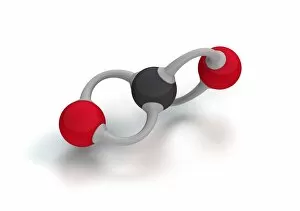
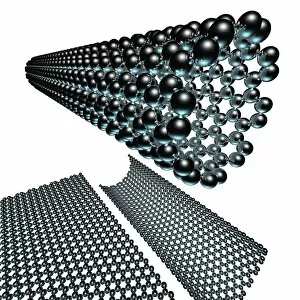
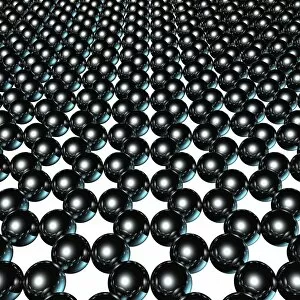
Carbon is the unsung hero of our modern world, with its versatility and strength making it an essential element in various industries. From the sleek Ducati 998R and 749R motorcycles made in Italy to the powerful Mercedes-Benz SL65 AMG Black Series, carbon fiber has revolutionized automotive engineering, providing lightweight yet durable components. But carbon's influence goes beyond just transportation; it extends into cutting-edge technology as well. With nanotube technology and graphene leading the way, scientists are unlocking new possibilities for electronics and materials. The intricate diamond computer artwork showcases how they are be transformed into a thing of beauty while retaining its exceptional properties. Art enthusiasts appreciate carbon's presence too. In Ellen Terry at Age Sixteen painting, we see how artists have used charcoal (a form of carbon) to create stunning portraits throughout history. Even contemporary art embraces this versatile element - just look at the captivating Graphene sheet artwork C016 / 8274. Not limited to man-made creations, nature also offers us glimpses of carbon's wonders. The Murchison CM2 carbonaceous chondrite meteorite holds secrets about our solar system's origins and provides valuable insights into organic chemistry. One cannot discuss carbon without mentioning its most famous form: the carbon nanotube. These microscopic structures possess incredible strength-to-weight ratios that could revolutionize fields like aerospace and medicine. Finally, we come full circle with Pagani Zonda C12s 3 and Pagani Zonda F supercars – both showcasing how high-performance vehicles benefit from incorporating advanced composite materials like carbon fiber reinforced polymers (CFRP). Whether it is propelling us forward on two wheels or four or pushing boundaries in science and art alike, there is no denying that "carbon" is a force to be reckoned with – transforming dreams into reality one atom at a time.